Our Journey
Friday 9 May 2025

This post outlines the development of our groundbreaking ultrasound system for non-invasive deep brain stimulation. We share the research journey from our academic origins to experimental validation, and how these advances led to founding NeuroHarmonics to create a wearable version of this technology for treating chronic brain disorders at home.
2016: Origins: Recognizing the Deep Brain Access Challenge
The story of NeuroHarmonics started in 2016, when members of our founding team were working as academic researchers at University College London. We recognized a fundamental limitation in treating brain related disorders: the inability to precisely target deep brain structures without invasive surgery. This was significant because many prevalent neurological conditions—including dementia, Essential Tremor, and depression—involve dysfunction in these subcortical regions. With over 3 billion people worldwide affected by neurological conditions and one in two people developing a central nervous system disorder by age 75, the lack of non-invasive treatment options for these critical brain areas represented both a massive unmet need and a compelling technological opportunity.
The scientific landscape was evolving rapidly. While several non-invasive brain stimulation techniques existed, their applications were largely limited to cortical regions near the skull. Other approaches either couldn't penetrate deeply enough or lacked the spatial precision needed for small subcortical targets. Recent breakthroughs had demonstrated that focused ultrasound could safely modulate neural activity in the human brain—a groundbreaking discovery that opened new possibilities for non-invasive neuromodulation. However, the technology to precisely target deeper brain structures with ultrasound remained undeveloped. Our team saw an opportunity to bridge this critical gap to create the first precision-targeted deep brain stimulation system without surgery.
With this vision, we secured funding from the Engineering and Physical Sciences Research Council (EPSRC) in a collaboration with the University of Oxford. Our multidisciplinary approach integrated three complementary areas of expertise: specialized ultrasound hardware development with low-cost wavefront shaping technologies; advanced computational acoustics through our open-source k-Wave modeling software; and deep neuroscience knowledge of brain circuits and neuromodulation. This unique combination of capabilities positioned us to overcome both the technical challenges of transcranial ultrasound delivery and the neuroscience requirements for effective, precise modulation of specific brain circuits—an achievement previously possible only through surgical intervention.
2021: Technical Innovation: Development of Precision Ultrasound Arrays
In 2021, after five years of intensive development, our team achieved a fundamental breakthrough with our multi-element transcranial ultrasound system. The technical challenge we faced was significant: the human skull strongly attenuates and distorts ultrasound waves, making it difficult to deliver sufficient energy to deep brain structures without affecting surrounding tissue. Additionally, many important brain targets are small (only a few millimeters across), requiring exceptional precision that conventional single-element ultrasound systems simply couldn't achieve.
Our solution was a 256-element transducer array arranged in a semi-ellipsoidal helmet design. Using multiple elements allowed us to control the timing and amplitude of each ultrasound wave independently, enabling them to arrive precisely synchronized at the intended target despite skull distortions. This multi-element approach also allowed us to create a much more focused beam than would be possible with a single transducer.
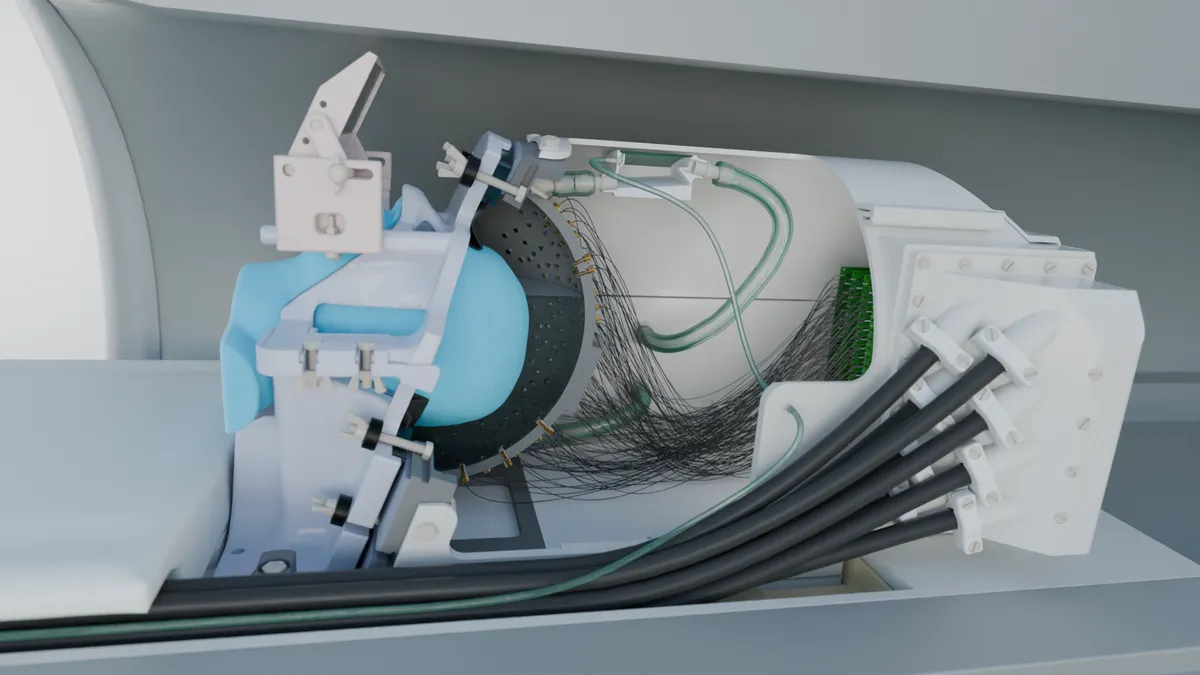
We developed several key innovations to make this system work effectively. First, we arranged the elements in a sparse, random pattern to prevent unwanted energy peaks (called grating lobes) that would otherwise stimulate unintended brain regions, compromising both safety and efficacy. Second, we implemented a custom-designed stereotactic positioning system using 3D-printed face and neck masks derived from individual MRI scans, ensuring precise alignment between the participant's brain and the ultrasound focus—critical when targeting structures just millimeters in size. Third, we created a computational acoustic model that accounted for each individual's unique skull geometry and tissue properties, enabling our system to compensate for the distortions caused by the skull.
Crucially, we designed the entire system to be MRI-compatible, allowing us to perform functional brain imaging simultaneously with ultrasound stimulation. This meant we could directly observe the neural effects of the stimulation in real time, providing immediate feedback on targeting accuracy and effectiveness.
The resulting system delivered unprecedented performance: a focal volume of just 3mm³, sub-millimeter targeting accuracy, and the ability to reach structures deep within the brain without affecting intervening tissue. This represented a 1,000-fold improvement in spatial precision over conventional ultrasound transducers and a 30-fold improvement over previous deep-brain targeting systems.
2023: Experimental Validation: Demonstrating Targeted Neuromodulation
In 2023, we conducted the first human studies to validate our system's ability to modulate neural activity in deep brain structures. We targeted the lateral geniculate nucleus (LGN)—a small thalamic structure involved in visual processing with a volume of approximately 80mm³, roughly the size of a small pea—while participants underwent functional MRI scanning.
A key innovation of our approach was the ability to stimulate the brain with ultrasound while simultaneously watching the brain's response using MRI. This real-time feedback was crucial in confirming that we were indeed affecting the exact brain regions we intended to target.
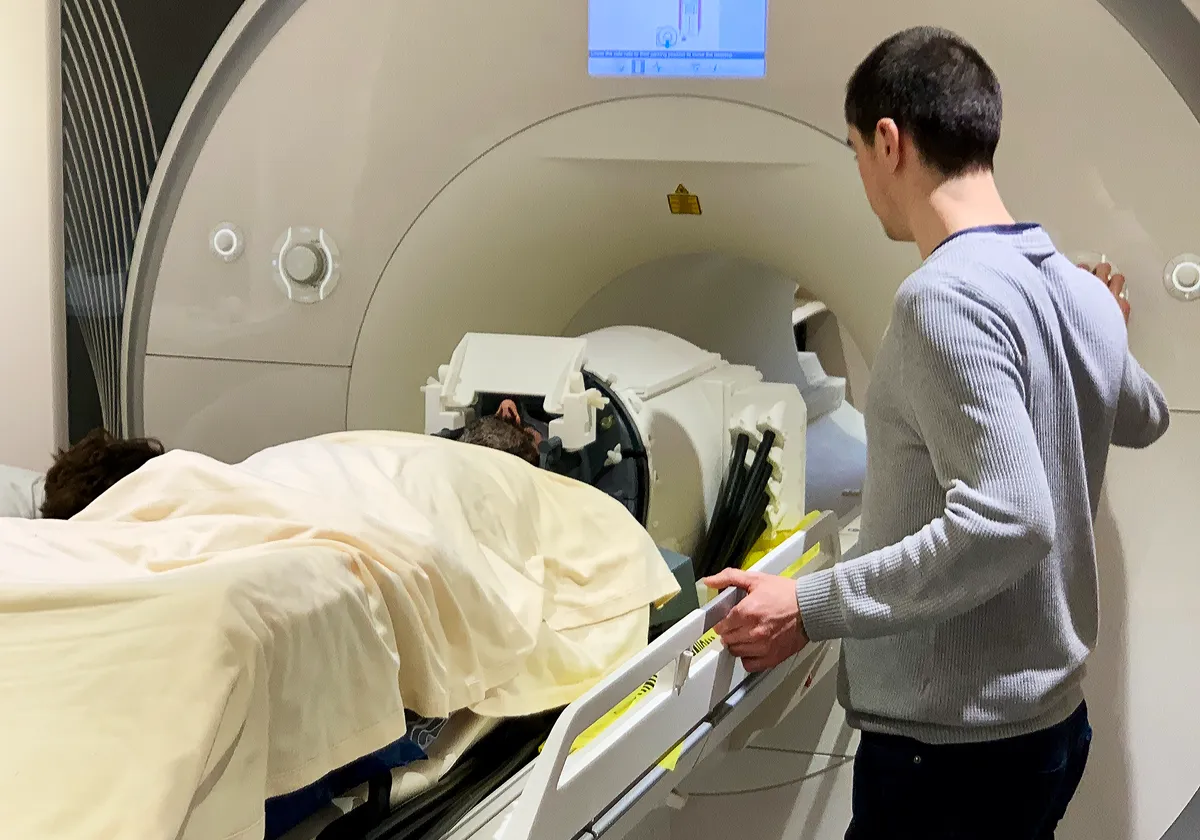
Research participant in our MRI-compatible ultrasound system during experimental validation of deep brain stimulation technology.
Using two complementary experimental designs, we demonstrated both immediate and lasting effects on brain activity. The online protocol delivered short ultrasound pulses during visual stimulation, allowing us to observe real-time effects. For the offline protocol, we used a "theta-burst" pattern—short pulses delivered rhythmically for 80 seconds—designed to induce longer-lasting changes in neural activity. Both approaches showed clear, measurable changes in brain activity in regions connected to the stimulated area.
The studies confirmed several crucial capabilities: precise targeting of structures as small as a pea deep within the brain, selective effects without affecting nearby regions, consistent results across different participants, and lasting effects for at least 40 minutes after a single stimulation session.
The demonstrated ability to precisely target such small, deep brain structures opened promising avenues for addressing conditions like essential tremor and treatment-resistant depression—disorders where dysfunction in specific deep brain regions plays a key role. For patients with these conditions, this could eventually mean access to non-invasive treatments that can modulate the exact neural circuits involved in their symptoms, potentially offering relief without medication side effects or surgical risks.
2024: Clinical Translation: From Laboratory Platform to Therapeutic Tool
With robust experimental evidence validating our approach and growing interest from the clinical neuroscience community, we recognized that translating this technology into widespread clinical use would require a fundamental redesign. While our MRI-integrated research system provided unprecedented spatial precision, its size, complexity, and cost made it impractical for regular therapeutic use, particularly for conditions requiring ongoing treatment. In December 2024, we established NeuroHarmonics to address this challenge, with a clear focus: developing a scaled-down, wearable ultrasound system practical for clinical and ultimately home use.
This transition presented substantial engineering challenges. We needed to miniaturize the system without compromising focal precision, develop a more user-friendly positioning approach that didn't require custom 3D-printed masks, and create treatment algorithms that could be safely administered without real-time MRI monitoring. Our computational models proved invaluable in this process, allowing us to simulate and optimize various hardware configurations to maintain focal accuracy while reducing the system's complexity.
Working closely with clinical collaborators at the University of Oxford, we're now planning pilot studies of our portable system in conditions where precise deep brain targeting holds particular promise, such as depression and essential tremor. Each represents a condition where current treatments are either ineffective for many patients or involve significant side effects and risks.
Our approach integrates several parallel streams of innovation. On the hardware side, we're addressing major barriers to practical home use, such as developing novel low-cost transducer arrays that maintain focal precision while dramatically reducing system complexity and cost. These hardware advances are complemented by continued refinement of our computational targeting models and development of protocols tailored to specific neural circuits.
The vision extends beyond any single condition. By creating this versatile neuromodulation platform, we aim to provide clinicians with a new therapeutic tool to address circuit-specific dysfunction across a range of neurological and psychiatric disorders—particularly for patients who haven't responded to conventional treatments. Our first-principles approach to solving the deep brain targeting challenge, validated through rigorous peer-reviewed research, positions us uniquely in this emerging therapeutic space. While significant work remains to validate these approaches in clinical settings, the foundational technology and initial human results suggest a promising path forward for non-invasive, accessible deep brain therapy that could transform treatment options for millions of patients.